Adjuvant for different vaccines
Since 2018 ASA is working in the field of pharmaceutical compounds produced by biotechnologal means.
In this process a GMP production process for the adjuvant c-di-AMP was successfully developed. The procedure is based on the enzymatical conversion of ATP to c-di-AMP by the DNA-integrity scanning protein (DisA):
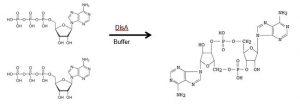
This adjuvant was combined in with different antigenes and tested in several toxicology studies. Since July 2023 a clinical trial phase I is running for the treatment of HPV.
Product data sheet: c-di-AMP